FTIR (Fourier Transform Infrared Spectroscopy) is a technique utilized for the identification of organic materials, polymers, and certain inorganic compounds. This analytical method is based on the interaction of infrared light with the sample. Additionally, FTIR is employed across various industries, including food, pharmaceuticals, and polymers, due to its ability to provide detailed information about molecular composition and structure.
How does FTIR work?
When infrared radiation is sent through a sample by the FTIR instrument, part of the energy is absorbed and some of it passes through. The sample molecules transform the absorbed radiation into bending (scissoring, rocking, wagging, and twisting) and stretching (symmetric and asymmetric) vibrational energy. The final signal at the detector is a spectrum that shows the sample’s molecular fingerprint. The spectrum fingerprints that each molecule or chemical structure produces are unique.
Infrared spectral region further divides into three ranges.
| Near IR (NIR) | Mid IR (MIR) | Far IR (FIR) |
| 4000 – 15000cm-1 | 400 – 4000cm-1 | 10 – 400cm-1 |
Measurement techniques in infrared spectroscopy
In FT-IR spectroscopy, three primary measuring methods can be employed: Transmission, Reflection, and Attenuated Total Reflection (ATR)
Transmission is the original method used in IR spectroscopy, where IR light passes through a sample to detect absorption. For effective transmission, the sample must be prepared properly; if too thick, it can lead to total absorbance, resulting in poor spectral quality with indistinct peaks. To prevent this, samples are diluted with an appropriate solvent (CCl4 for liquids) or mixed with KBr for solids, which is then pressed into a pellet or placed on a KBr window.
Infrared (IR) light is reflected from the surface of the sample and is subsequently detected by a sensor. This method is commonly employed for the analysis of solid samples.
ATR (Attenuated Total Reflectance) has become the primary technique in IR spectroscopy due to its minimal sample preparation and non-destructive nature. In ATR, the sample is placed on a crystal (usually made of diamond, germanium, or zinc selenide), and IR light is directed through the crystal, interacting only with the top few microns of the sample. This approach eliminates the need for extensive sample preparation, resulting in high-quality spectra regardless of the sample type.
Sample technique
- Gas sample – Gas Cell
- Liquid sample – Liquid Cell, Attenuated Total Reflectance (ATR)
- Powder samples – KBr pellets, ATR
Sample Preparation methods
- Grind the sample into fine particles using a mortar and pestle.
- Thoroughly mix the ground sample with potassium bromide (KBr) in a ratio of approximately 1% sample to 99% KBr.
- Prepare a transparent disk by pressing the mixture using a hand press.
- Molecular distortion may occur during the high-pressure preparation of the disk, leading to changes in morphology.
- A sloping baseline in the spectrum can result from uneven particle size distribution.
- Incomplete drying of potassium bromide (KBr) may also affect the quality of the sample.
- Dissolve the sample in an appropriate solvent.
- Pour the solution onto a flat infrared window.
- Allow the solvent to evaporate completely.
- Measure the resulting film using transmission spectroscopy.
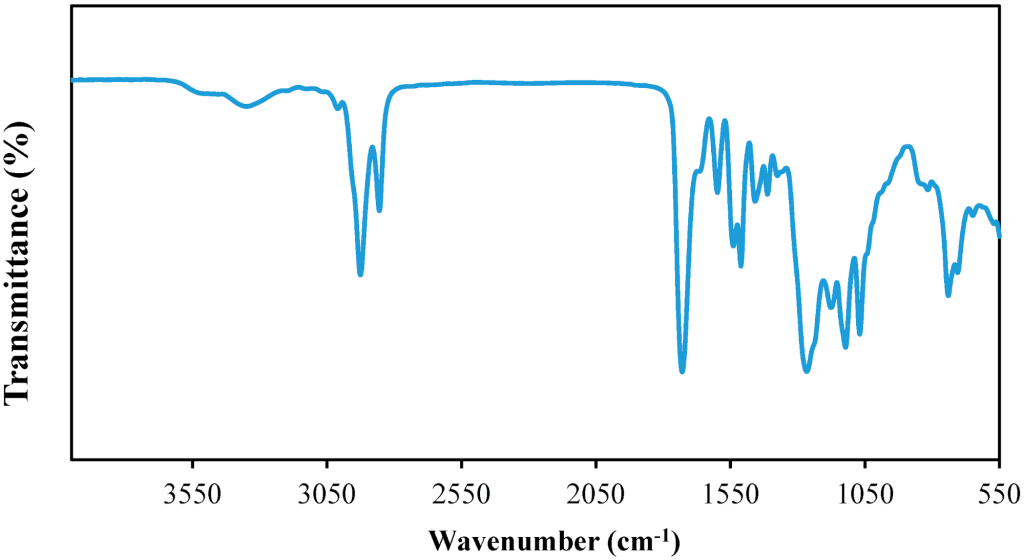
Specific IR bands
| Wave number cm-1 | Bond | Functional group |
| 3200 – 3600 | O-H | Alcohol |
| 1650 – 1750 | C=O | Carbonyl |
| 1700 – 1725 | C=O | Carboxylic acid |
| 2500 – 3300 | O-H | Carboxylic acid |
| 1620 – 1680 | C=C | Alkene |
| 2100 – 2250 | C≡C | Alkyne |
| 3300 – 3500 | N-H | Amine |
Advantages of FTIR
- The sample spectrum can be obtained in just a few seconds.
- This method offers higher sensitivity.
- It is a non-destructive technique.
- Only a small amount of sample is needed for analysis.
- A wide variety of samples can be analyzed using this method.
Application of FTIR
- Material Characterization: Identifying and characterizing polymers, plastics, and other materials by analyzing their molecular structures.
- Pharmaceutical Analysis: Determining the composition and purity of pharmaceutical products.
- Environmental Testing: Monitoring pollutants in air, water, and soil by identifying chemical contaminants.
- Food Quality Control: Analyzing food products for authenticity, contamination, and nutritional content.
- Forensic Science: Identifying substances in forensic samples, such as drugs or explosive residues.
- Biological Research: Studying biomolecules, including proteins and nucleic acids, to understand their structure and function.
- Coatings and Thin Films: Analyzing surface coatings, including paints and varnishes, to understand their chemical properties.
Maintenance for FTIR instrument
- Fourier Transform Infrared Spectrometers contain costly hygroscopic components, necessitating storage in a completely moisture-free environment.
- Renew the desiccant at the specified intervals.
- Schedule desiccant changes during the driest times of the year.
References
Read more…
